According to the Office of the Surgeon General, 1.5 million Americans suffer fractures from bone disease every year. In experiments in rats and human cells, Johns Hopkins Medicine researchers say they have added to evidence that a cellular protein signal that drives both bone and fat formation in selected stem cells can be manipulated to favor bone building.
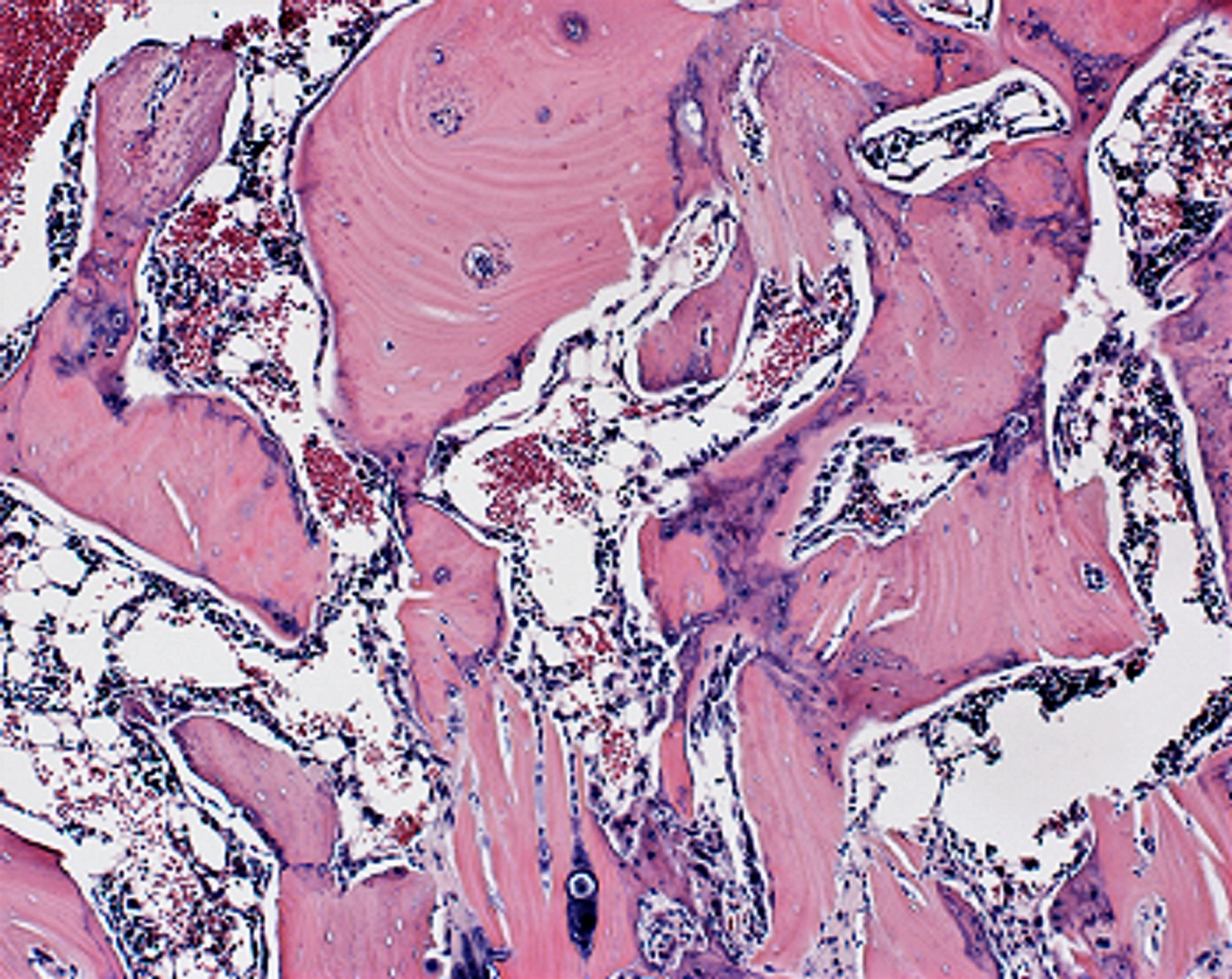
Our bones have a limited pool of stem cells to draw from to create new bone, says Aaron James, M. D., associate professor of pathology at the Johns Hopkins University School of Medicine, and the study’s senior author. Such a procedure requires a massive amount of new bone cells, says James.
These are embryonic-type stem cells. After four weeks, the researchers studied the rats’ spinal tissue and observed continued high levels of the WISP-1 protein. They also observed new bone forming, successfully fusing the vertebrae together, whereas the rats not treated with stem cells making WISP-1 did not show any successful bone fusion during the time the researchers were observing.
Johns Hopkins researchers have identified a protein involved in cell proliferation and the development of new blood vessels that could serve as a marker. These results were published online in the Nov. 28 issue of the journal Biochemical and Biophysical Research Communications. Despite the fact that adult stem cells have already shown documented evidence not only of their safety, but of their efficacy at treating spinal cord injury.
In a new study published in Nature, scientists from Bart Deplancke’s lab at EPFL, Christian Wolfrum’s lab at ETHZ, and the Swiss Stem Cell Foundation led by Gianni Soldati, have used a high – resolution technique called ” single cell transcriptomics ” to characterize, for the first time, the different types of stromal cells that reside within mature fat depots. Interestingly, their gene expression was similar to fat cells. “Our work shows that there are still many human cell types that await discovery”, says Christian Wolfrum.
We show that young macrophage cells produce factors that lead to bone formation, and when introduced in older mice, improves fracture healing, said Gurpreet Baht, Ph. D., assistant professor in orthopedic surgery and a lead author of the study.
Researchers at Texas A&M University have explored a new class of nanoparticles that can direct stem cells to become bone or cartilage cells without the use of these growth factors. Generating induced pluripotent stem cells from large numbers of patients is expensive and laborious.
People with Parkinson’s disease have a higher risk of broken bones. According to the lunginstitute.com, a handful of clinical trials in the U. S. use stem cell treatment to promote healing in those challenged with these diseases.