A new catalyst could dramatically decrease the cost of producing hydrogen, one of the cleanest renewable fuels. Now, researchers say the metal could advance one of the most promising sources of renewable energy: hydrogen fuel cells. There’s a new contender in the race to find an inexpensive alternative to platinum catalysts for use in hydrogen fuel cells. ” Like a battery, hydrogen fuel cells convert stored chemical energy into electricity. Taking part in the worldwide search for fuel cell cathode materials, researchers at the University of Akron developed a new method of synthesizing catalysts from a combination of metals-platinum and nickel – that form octahedral ( eight-sided ) shaped nanoparticles. While scientists have identified this catalyst as one of the most efficient replacements for pure platinum, they have not fully understood why it grows in an octahedral shape.
To better understand the growth process, the researchers at the University of Akron collaborated with multiple institutions, including Brookhaven and its NSLS-II. “The growth process case for the platinum-nickel system is quite sophisticated, so we collaborated with several experienced groups to address the challenges”. The DOE Science News Source is a Newswise initiative to promote research news from the Office of Science of the DOE to the public and news media.
In a study published Oct. 29, 2018 in the journal Nature Catalysis, a University at Buffalo – led research team reports on catalysts made from the widely available and inexpensive metal. “We haven’t been able to advance a large-scale hydrogen economy because of this issue involving catalysts. Platinum has been the king of the catalysts, for the making of electricity in hydrogen-powered fuel-cells, for many years. The goal, William Studier, a Senior Biophysicist Emeritus at the U. S. Department of Energy’s Brookhaven National Laboratory and Adjunct Professor of Biochemistry at Stony Brook University says, is to further enhance the catalyst’s performance in practical hydrogen fuel cells. He, has been elected as a Fellow of the National Academy of Inventors ( NAI ).
In a new study, Argonne scientists have created small regions of magnetic defects. When electromagnetic plane waves interact with these defects, they are converted into helical waves, which encode more information for further materials studies. Scientists at Oak Ridge National Laboratory have created a recipe for a renewable 3D printing feedstock that could spur a profitable new use for an intractable byproduct: lignin. A team of scientists has for the first time measured the elusive weak interaction between protons and neutrons in the nucleus of an atom.
Through a unique neutron experiment at Oak Ridge National Laboratory, experimental physicists resolved the weak force between the particles at the atom’s core, predicted in the Standard Model that describes the elementary particles and their interactions. A discovery by researchers at the U. S. Department of Energy’s Lawrence Berkeley National Laboratory and the Joint Center for Artificial Photosynthesis shows that recycling carbon dioxide into valuable chemicals and fuels can be economical and efficient – all through a single copper catalyst. An experiment that aims to gain new insight into the force that binds all matter together has recently completed its first phase of data collection.
The Gluonic Excitations Experiment, or GlueX, is designed to produce and study hybrid mesons, which are particles that are built of the same stuff as ordinary protons and neutrons: quarks bound together by the ” glue ” of the strong force. But unlike ordinary mesons, the glue in hybrid mesons behaves differently by actively contributing to the particles’ properties. The U. S. Department of Energy has approved the technical scope, cost estimate and plan of work for an upgrade of the Advanced Photon Source, a major storage-ring X-ray source at Argonne. A breakthrough in the Lab’s development of UCNPs was in finding ways to boost their efficiency in emitting the absorbed light at higher energies, said Emory Chan, a staff scientist at the Molecular Foundry who also participated in the latest study.
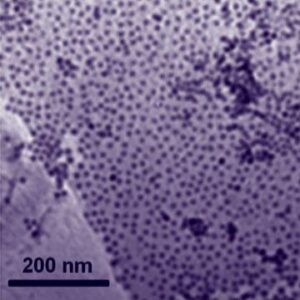 A grant from DOE’s Technology Commercialization Fund will help researchers at Argonne and industry partners seek improvements to U. S. manufacturing by making discovery and design of new materials more efficient. The new material “has the best reduction performance of any nonplatinum catalyst” , said Shaojun Guo, postdoctoral researcher in Sun’s lab and lead author of a paper published today in the journal Angewandte Chemie International Edition. Sun and his team used a self-assembly method that gave them more control over the material’s properties. This usually happens at very low temperatures. The results of their study were published in Physical Chemistry Chemical Physics.
A grant from DOE’s Technology Commercialization Fund will help researchers at Argonne and industry partners seek improvements to U. S. manufacturing by making discovery and design of new materials more efficient. The new material “has the best reduction performance of any nonplatinum catalyst” , said Shaojun Guo, postdoctoral researcher in Sun’s lab and lead author of a paper published today in the journal Angewandte Chemie International Edition. Sun and his team used a self-assembly method that gave them more control over the material’s properties. This usually happens at very low temperatures. The results of their study were published in Physical Chemistry Chemical Physics.
In the quest to replace fossil fuels, scientists are always on the lookout for alternative, environmentally friendly sources of energy. It sounds like something straight out of Alice in Wonderland, but researchers have now generated mushrooms patterned with energy-producing bacteria and an electrode network. Depending on the lighting, the surface of appropriately crafted nanoparticles can change its topography. The most apparent roadblocks are – as is true for most new technologies – cost and efficiency. In our method, the catalyst is deposited on nanoparticles. Researchers from the IPC PAS are still looking for ideas on how their concept could translate into specific applications.
Physicists from the University of California, Irvine have discovered a new way to control magnets at the nanometer scale by electric current. Researchers have developed what is described as the world’s most efficient organic solar cell. The cell is formed from a new type of organic molecule and energy capture results from a prototype are described as “impressive”. The new material is also very durable. The bio-brick process produces as by-products nitrogen and potassium, which are important components of commercial fertilisers. The Department of Chemistry, IIT Jodhpur’s new research has found a way to break Oxygen and Hydrogen molecules in water by using a catalyst called Lanthanide. The head of the Chemistry department at IIT-Jodhpur, Rakesh Kumar Sharma, also a leader in this project says that his researchers tried over 700 different types of catalyst combination to obtain the pure hydrogen. That in fact nuclear energy can efficiently provide a far greater share of American electric generation.

